WHAT IS FRAGILE X SYNDROME?
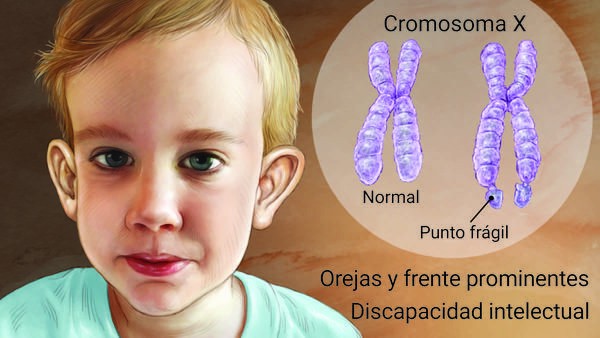
Fragile X syndrome (FXS) is the most common inherited cause of mental retardation with approximately 1 in 4,000 men affected. In the vast majority of cases, this X-linked disorder is caused by CGG repeats in the 5′ untranslated region (UTR) of the FMR1 gene.
FXS-causing alleles, or full-length mutations, contain 200 or more copies of the repeat that are hypermethylated and transcriptionally silenced. Unstable alleles that give rise to full mutations are called premutations and are associated with phenotypes other than FXS. The mutational mechanism, combined with the location of this gene on the X chromosome, leads to remarkable inheritance patterns in which the relevant alleles are transmitted from intellectually normal men through their unaffected daughters and then to affected sons.
FXS can be suspected in both sexes and includes a variable clinical phenotype. People with FXS can range from learning disabilities and normal IQ to severe mental retardation and autistic behaviors. Physical characteristics have been described, but are often not specific. Thus, the diagnosis is made based on the detection of alterations in FMR1.
Clinical features
The physical features of FXS are quite subtle and the first clinical indication is usually developmental delay, mild motor delays and/or language delays. Autistic-like behaviors such as hand flapping, poor eye contact, and hand biting may be seen.
The average IQ in adult males with the full, fully methylated mutation is approximately. Less affected men often have incomplete methylation, resulting in incomplete activation of FMR1, and may even have an IQ in the borderline or low normal range. In general, for FXS, cognitive deficits include problems with short-term and working memory, executive function, and math and visuospatial skills. Because the disorder is X-linked, women are generally much more mildly affected than men, particularly in terms of cognitive functioning, but tend to be at higher risk for emotional problems compared to the general population. Women with the full mutation typically have normal or borderline IQs, and most will have associated learning disabilities and/or emotional problems.
People with FXS do not usually have significant medical problems. Recurrent otitis media and recurrent sinusitis are common during childhood. There may be joint laxity with hyperextensibility in the toe joints and flat feet, which usually improves with age. Gastroesophageal reflux disease occurs in one-third of young infants with FXS and may present with recurrent irritability or emesis. Seizures and epilepsy are another common feature of FXS during childhood, with an incidence of 13-18% in boys and 5% in girls.
Most people with the premutation have normal intelligence, but males are prone to attention problems, executive dysfunction, social deficits, and obsessive-compulsive behavior. Approximately 20% of women who have an FMR1 premutation have premature ovarian failure (POF), which is the premature cessation of menstruation before the age of 40.
Behavioral aspects
The behavioral phenotype may be helpful in suggesting the diagnosis of FXS. Autistic-like features are common in people with FXS and include hand flapping, hand biting, gaze avoidance, tactile defensiveness, and hyperarousal to sensory stimuli. These features, along with impaired social skills such as socioemotional reciprocity, are expressed to varying degrees in children with FXS and may be indicative of a concurrent diagnosis of autism spectrum disorder or autism-like behavior. Anxiety and mood disorders, hyperactivity, impulsivity, and aggressive behavior may also occur.
The emotional and behavioral characteristics of women with FXS are often variable. Women with the full mutation are prone to social anxiety, shyness, social avoidance, withdrawal, language deficits, mood lability, and depression. In addition, women with the premutation have also been reported to have social anxiety.
physical characteristics
Physical features include macroorchidism that is evident just before puberty and those related to connective tissue dysplasia, including a long narrow face, prominent ears, joint hypermobility, and flat feet. Facial features can be subtle and may become more apparent with age.
Molecular bases of the disease
In 1991, the gene responsible for FXS, FMR1, was identified. Fragile X was the first known example of a trinucleotide repeat disorder.
There are four allelic classes for the CGG repeat tract in the 5′-UTR of FMR1. The repeat sizes for each group are not well defined and this complicates genetic counseling. In the general population, the repeat tract contains up to 40 repeats, with 30 being the most common (normal or common alleles). Then there are the intermediate alleles, which range from 41 to 54 repeats and are generally not associated with repeat tract instability.
Full mutations, which cause FXS, have more than 200 copies of the repeat.
Diagnosis
It is accepted practice to order Fragile X testing in all children with developmental delay, mental retardation, or autism, although this may have a diagnostic yield of only approximately 1-2%. The presence in the proband’s family of movement disorders, learning disabilities, mental retardation, or primary ovarian insufficiency should raise suspicion of the presence of FMR1 mutations in the family.
The test procedure comprises two complementary analyses. PCR with repeat-flanking primers is used to determine the number of CGG repeats in the FMR1 5′-UTR, and a Southern blot of genomic DNA is used to determine methylation status and measure the size of full-length mutations, which are often are resistant to PCR amplification. The combination of Southern blot and PCR for the detection of mutations associated with Fragile X has a sensitivity of 99%.
Genetic counseling
If a positive FXS test is discovered, the patient and family should be referred for genetic counseling and testing of family members at risk of carrying a full mutation or premutation. Carriers of the premutation should be counseled about the risks of passing a full mutation to their children.
When planning tests in an affected family, special attention should be given to family members with mental retardation, learning disabilities, autism, or social and behavioral disorders; female relatives with infertility or premature menopause; and those with tremor, ataxia, or other neurological and psychiatric problems.
Treatment and care
Psychopharmacological intervention should be combined with other supportive strategies, including speech therapy, sensory integration occupational therapy, individualized educational plans, and tailored behavioral interventions to maximize functioning.
In children with FXS, the most commonly used medications are stimulants. These medications target the symptoms of hyperactivity, impulsivity, and distractibility and can be very helpful in these areas. Despite being the most common medication in FXS, the effectiveness of these medications and their side effects vary for each individual. The response rate to stimulants may be relatively lower in adult men with FXS due to their higher anxiety and lower activity level.
AUTHOR: M.C. KAREM DANIELA FAUSTO LIZÁRRAGA
Bibliography
- Crawford, D. C., Acuña, J. M., & Sherman, S. L. (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in medicine, 3(5), 359-371.
- De Vries, B. B., Halley, D. J., Oostra, B. A., & Niermeijer, M. F. (1998). The fragile X syndrome. Journal of medical genetics, 35(7), 579-589.
- Freund, L. S., Reiss, A. L., & Abrams, M. T. (1993). Psychiatric disorders associated with fragile X in the young female. Pediatrics, 91(2), 321-329.
- Garber, K. B., Visootsak, J., & Warren, S. T. (2008). Fragile X syndrome. European journal of human genetics, 16(6), 666-672.
- Hagerman, R. J., Berry-Kravis, E., Hazlett, H. C., Bailey, D. B., Moine, H., Kooy, R. F., … & Hagerman, P. J. (2017). Fragile X syndrome. Nature reviews Disease primers, 3(1), 1-19.
- Hagerman, R. J. (2005). Fragile X syndrome. Management of genetic syndromes, 251-263.
- Kemper, M. B., Hagerman, R. J., & Altshul‐Stark, D. (1988). Cognitive profiles of boys with the fragile X syndrome. American journal of medical genetics, 30(1‐2), 191-200.
- McLennan, Y., Polussa, J., Tassone, F., & Hagerman, R. (2011). Fragile x syndrome. Current genomics, 12(3), 216-224.
- Merenstein, S. A., Sobesky, W. E., Taylor, A. K., Riddle, J. E., Tran, H. X., & Hagerman, R. J. (1996). Molecular‐clinical correlations in males with an expanded FMR1 mutation. American journal of medical genetics, 64(2), 388-394.